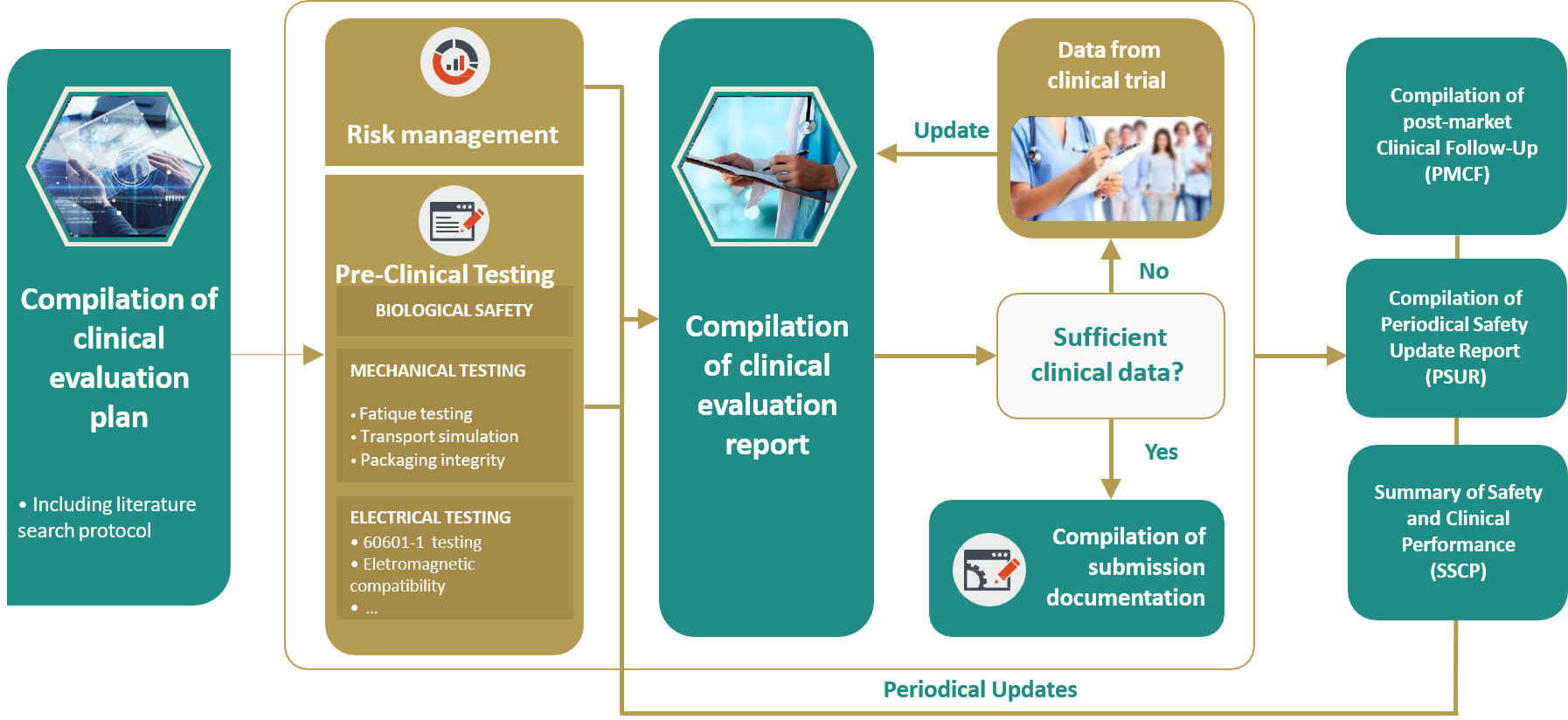
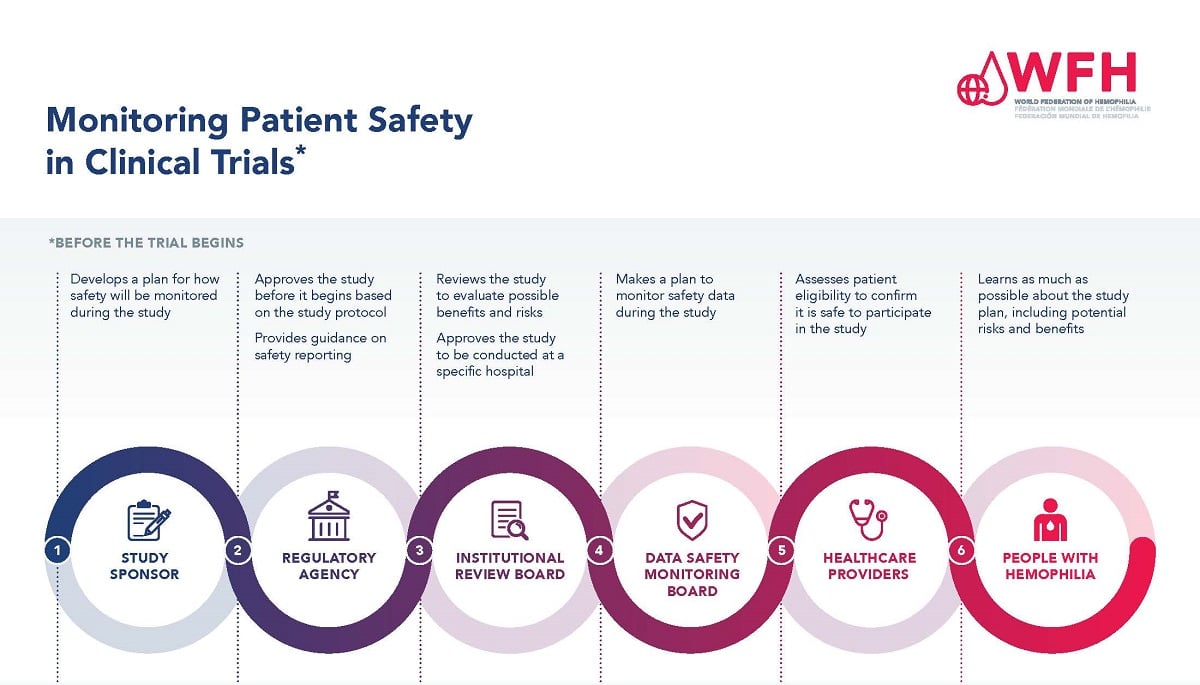
Global Healthcare Brand Improves Safety Reporting in Clinical Trials Leveraging Pharmacovigilance Analytics| Quantzig | Business Wire

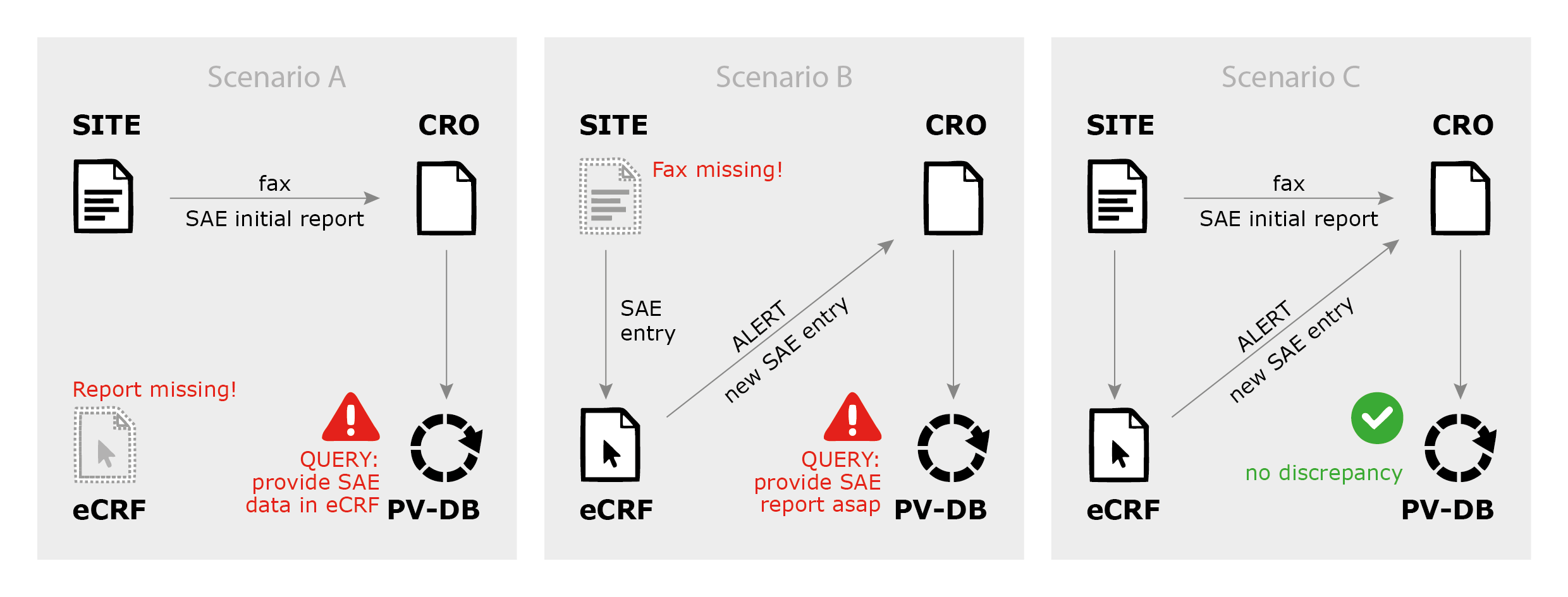
1 Nuts and Bolts of Safety Reporting The Role of the CRO Dr. Noa Lowenton Spier Pharma-Clinical S.A.G. - ppt download